Molecular Pathology

comprehensive specialised molecular oncology
Providing predictive, germline & somatic mutation analyses.
At nexomics we offer state-of-the-art molecular pathology services on multiple high-throughput test platforms that can interrogate RNA, DNA, ctDNA and protein from a large number of samples simultaneously.
We provide clinicians with a personalised and user friendly reporting that outlines the genomic findings and patient treatment options.
Our team regularly partners with collaborators to design clinical grade tumour specific assays to support patient care and clinical research with an emphasis on exploratory biomarker studies within small and large scale clinical trials.


complimentary genomics SERVICES
Chromogenic and fluorescent in-situ hybridisation (FISH) Services
Chromogenic and fluorescent in-situ hybridisation (FISH) is a powerful laboratory technique used to detect and localise specific DNA or RNA sequences directly within cells or tissue sections using fluorescent probes (FISH) or chromogenic based detection (CISH).
This molecular analysis method, which provides the precise localisation and quantification of nucleic acids such as DNA, mRNA, and microRNA, is a powerful tool in cancer for the analysis of genetic abnormalities.
FISH and CISH technologies provide complementary genomic information to NGS,NanoString, qPCR, and ddPCR techniques using both FFPE and blood samples.

COMPREHENSIVE genomics SERVICES with high sensitivitY & Absolute quantification
Real-time PCR, qPCR and ddPCR Services
Nexomics offer a range of real-time PCR, quantitative PCR (qPCR) and droplet digital PCR (ddPCR) services as part of our comprehensive molecular pathology offering.
Utilising our Bio-rad ddPCR and ABI 7500 Fast qPCR platforms we can support your clinical trial project with bespoke testing solutions utilising a wide range of sample types.
Our team of experts can assist with the following qPCR and ddPCR services:
- Variant detection
- Gene expression
- Copy number variation (CNV) detection

Quantitative PCR (qPCR)
Quantitative PCR (qPCR) plays a critical role in cancer diagnosis and monitoring by detecting and quantifying specific genetic changes associated with cancer. It's valued for its high sensitivity, speed, and ability to detect very low levels of abnormal DNA or RNA in patient samples.
Droplet Digital™ PCR (ddPCR™)
Droplet Digital™ PCR (ddPCR™) offers highly sensitive nucleic acid detection and precise quantification. It excels in identifying low-abundance targets, such as allelic or structural variants, which are often undetectable by other platforms. With its advanced multiplexing capabilities, ddPCR technology enhances the analysis of multiple targets per well while maintaining high performance.
SPECIALISED LIQUID BIOPSY FOR PRECISE MONITORINGOF TUMOUR DNA
Liquid Biopsy Testing
Liquid biopsy is a minimally-invasive testing method that detects cancer-related material in body fluids, including blood, urine, saliva, or cerebrospinal fluid (CSF).
Liquid biopsies provide a less invasive sample collection process than traditional tissue biopsies and can be used to analyse:
- Circulating tumour DNA (ctDNA)
- Circulating tumour cells (CTCs)
- Exosomes
- RNA or proteins associated with cancer

Oncomine Precision Panel (OPA)
Cutting-edge testing leverages minimally-invasive liquid biopsy technology to detect and monitor tumour-specific biomarkers in blood providing early cancer detection, monitoring treatment effectiveness, detecting recurrence, identifying genetic variants to guide targeted therapies and tracking of tumour evolution and resistance to treatment.
Unlock Precision Oncology with the Ion Torrent Genexus System
This next-generation solution delivers rapid, comprehensive genomic profiling, detecting critical biomarkers like EGFR, ALK, BRAF, ROS1, NTRK, RET, and ERBB2 from FFPE tissue or liquid biopsy specimens.
- Mutation, CNV, and fusion variant types across 50 genes including EGFR, ALK, BRAF, ROS1, RET, KRAS, PIK3CA.
- Compatible with FFPE tissue as well as liquid biopsy samples.
Enabling precision oncology through SEQUENCING insights
Sequencing Services
We are committed to providing exceptional service and innovative solutions that empower our clinical partners. From clinical trials to bespoke assay development, our team supports every stage of clinical development with specialised molecular oncology and haematological solutions.
nexomics are equipped with a variety of different sequencing platforms including the Applied Biosystems ABI 3730xl DNA Analyzer which is a high-throughput genetic analysis instrument designed for various applications, including DNA sequencing and fragment analysis.
Our NGS sequencing platforms include Illumina NovaSeq6000, Illumina NextSeq500 and Thermo Fisher Ion Torrent Genexus system allowing us to provide NGS testing solutions that accommodate a wide range of throughput and batching requirements.


Familial Cancer Predisposition
Comprehensive genetic testing to identify inherited mutations linked to cancer pre-disposition syndromes. Empowering clinicians and patients with actionable insights, our testing supports risk assessment, preventive strategies, and personalised care for hereditary cancers.
The following clinically focussed gene panels:
In addition to our genepanels, we offer targeted predictive, confirmatory and segregation testing forknown familial variants as well as origin of variant (germline v somatic)testing for variants detected in tumour samples.
- Ovarian
- Prostate
- Pancreas
- Breast, Ovarian,Prostate and Pancreas
- Colorectal andEndometrial
- Mismatch Repair (MMR)
- Polyps
- Renal
- Paraganglioma,Pheochromocytoma & GIST
- Pituitary
- Melanoma
- Gorlin Syndrome
- Facial PapuleSyndrome
- Schwannomata’s
- Sarcoma
- Haematological Malignancy Predisposition
- Endocrine

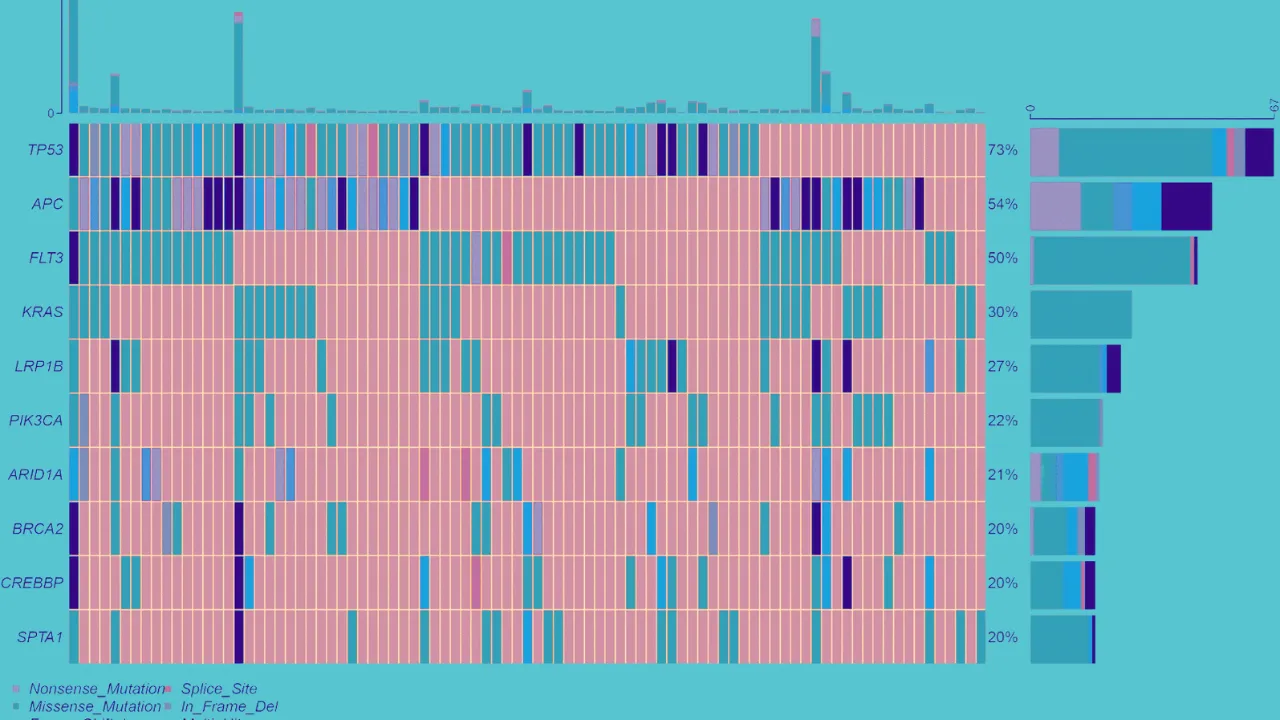
Molecular Oncology
Advanced somatic testing focuses on identifying tumour-specific mutations, enabling precise tumour profiling and targeted therapy selection. Our state-of-the-art capabilities provide critical data for personalised cancer treatment and clinical trial enrolment.
Utilising our Thermo Fisher Ion Torrent Genexus System we offer formalin-fixed paraffin-embedded (FFPE) tissue testing using the Oncomine Precision Assay (OPA). The OPA includes mutation, CNV, and fusion variant detection across 50 key genes (including EGFR, ALK, BRAF, ROS1, RET, KRAS, PIK3CA, and ERBB2):
- Melanoma
- Colorectal
- NSCLC
- GIST
- Breast
- Thyroid
- Brain
- Ovarian HRD/HRR
- Sarcoma

Molecular Haematology
Supporting integrated haematology diagnostic solutions for blood cancers, including leukaemia, lymphoma, and myeloma.
Led by Associate Professor Piers Blombery, the Wilson Centre for Blood Cancer Genomics at Peter Mac provides accredited comprehensive genomic testing for patients with blood cancer to uncover the molecular characteristics of their cancer and improve diagnosis, treatment and outcomes.
Nexomics, through our partnership with the Department of Pathology and the Wilson Centre for Blood Cancer Genomics at Peter Mac, can offer a range of haematological assays including comprehensive DNA (80 gene) and RNA (71 gene) NGS panels as well as a Myeloproliferative Neoplasm (MPN) specific 22 gene NGS panel. In addition, we offer the following testing.
- Adaptive clonoSEQ IGH/IGK/IGL MRD
- IGHV Somatic Hypermutation (SHM)
- FLT3-ITD & TKD (non-quantitative)
- NPM1 (non-quantitative)
- t(9;22) BCR: ABL1 (p210, p190 fusion transcripts)
- NPM1 MRD (Type A/B/D)