Clinical Trial Services and Support

a centralised hub FOR ONCOLOGY CLINICAL TRIALS TESTING
Optimising diagnostic workflows delivering clinical trial testing with efficiency and precision.
At nexomics, we assist our partners with tailored clinical trial testing and management solutions. This includes standardised clinical trial specific laboratory manuals, sample collection kits and test requisition forms bringing efficiency and precision to the sample collection process for all clinical trials scenarios.
Project Management FROM STUDY INCEPTION THROUGH TO CLOSURE.
A Partner in Study Management & Clinical Research.
We at nexomics offer dedicated study and project-specific management support and oversight. Our team comprises a diverse array of scientific, technical, operational, and business development experts.
- Study specific project management
- Teleconferences and study start-up activities
- Dedicated scientific leads assigned to each study ensuring personalised service and seamless communication with regular study updates including testing and reporting progress.
- Analytical, verification and validation plans and reports
- Clinical sample and collection kit tracking, logistics and management
- Data transfer setup and management

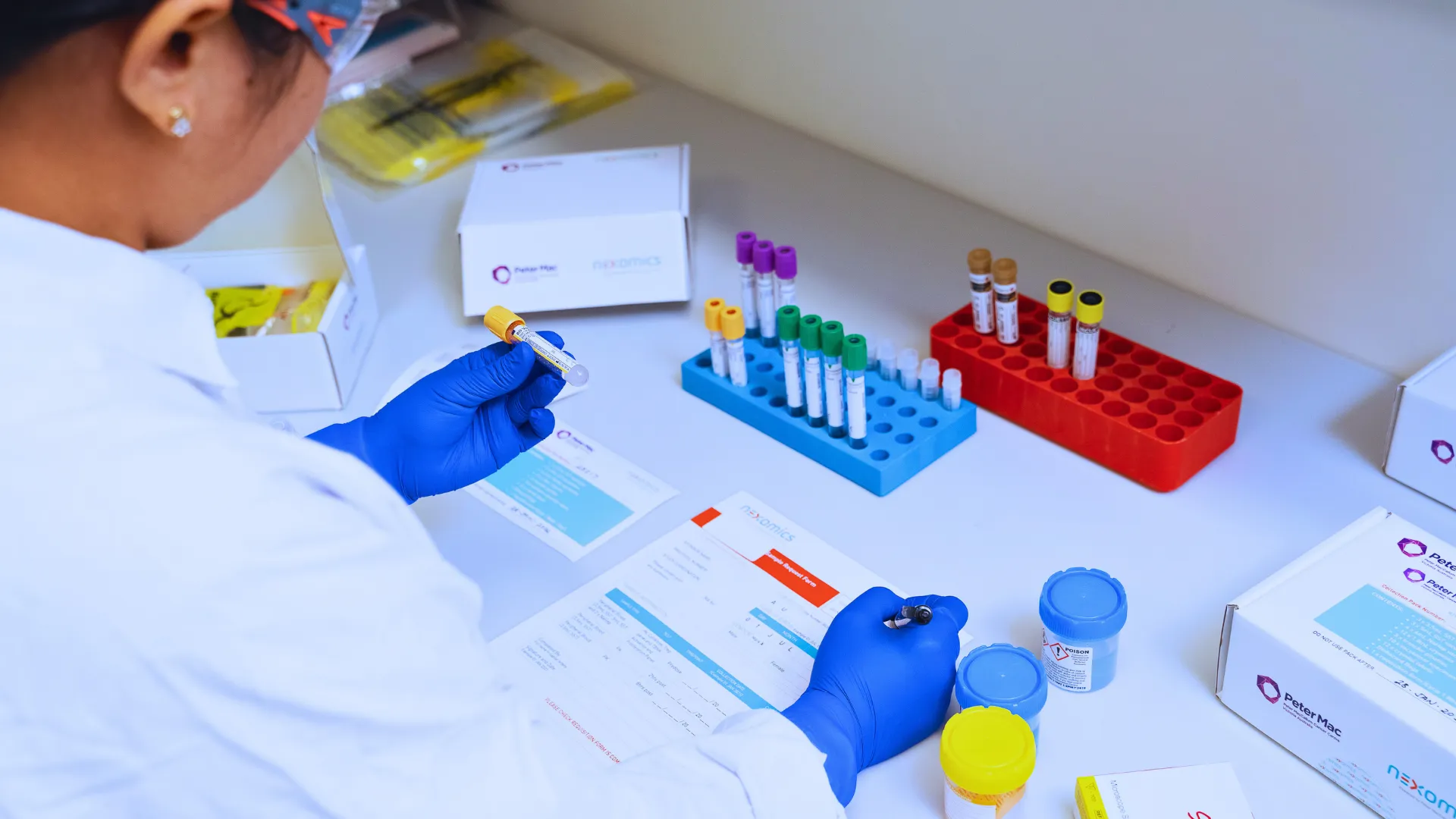
SEAMLESS SAMPLE MANAGEMENT
Every sample matters. Every patient counts.
Our team is responsible for overseeing the entire sample workflow ensuring:
- Our team is responsible for overseeing the entire sample workflow.
- We ensure efficient management of each sample from initial sample receipt through to facilitating prompt sample processing, transfer to the appropriate downstream department or laboratory for further clinical trial or bespoke testing and temperature appropriate storage to preserve sample integrity.
- We ensure the safe shipment of your samples nationally and internationally for further testing or banking at a facility of your choice.
- Our team excels in managing a wide variety of samples for further testing, including liquid biopsies, tissue biopsies (formalin-fixed and/or paraffin-embedded (FFPE) sections, FFPE blocks, frozen and fresh tissue) and cryopreserved cells.
- We possess comprehensive knowledge of the specific requirements for sample processing, storage and testing across all pathology disciplines, such as anatomical pathology, immunology, and molecular pathology.

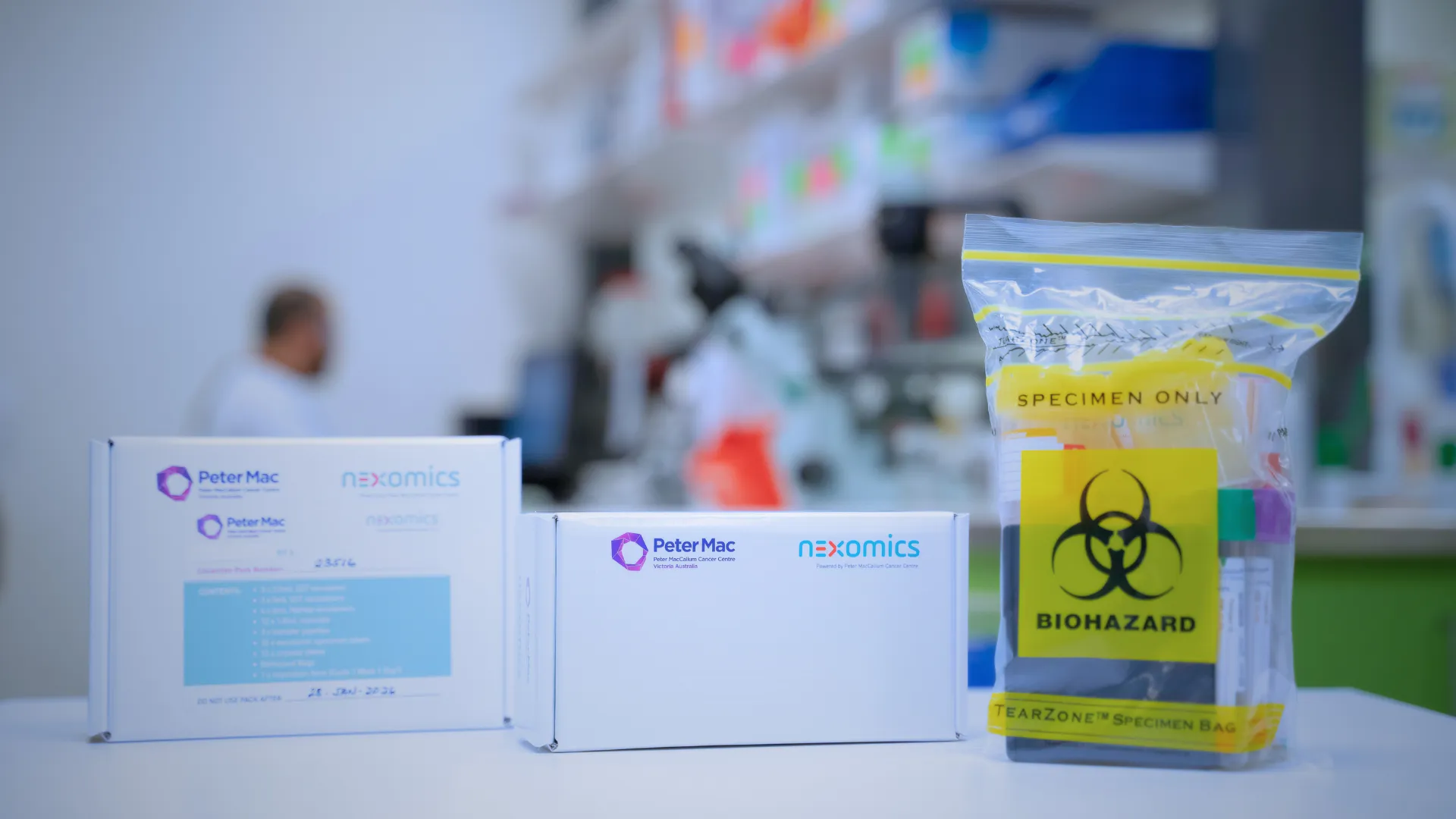
CENTRALISED COLLECTION KIT PRODUCTION
Streamlined for efficient sample collection.
Collection Kit Production and Supply
nexomics simplifies sample collection for clinical trials by providing comprehensive, protocol-specific collection kits on a global scale.
- We design and assemble sample collection kits, including consumables, labels, and requisition forms, tailored to your study's needs.
- We create precise lab manuals with detailed sample collection, storage, and shipping procedures tailored to your study.
- We ensure timely delivery of collection kits by navigating global shipping regulations and import permits, working closely with couriers and customs.
comprehensive instruction for effecient collection.
Trial Success. Precise Instruction.
nexomics provides comprehensive laboratory manuals detailing study-specific procedures for sample collection, storage and shipment to our facility at the Peter Mac to ensure consistent and efficient clinical trial sample processing, labelling, testing and shipment.
- Ensuring compliant records and sample integrity for optimal testing conditions.
- Adhering to standardised procedures minimises variability, ensuring reliable results and efficient sample transport while maintaining data integrity for regulatory needs.
- Nexomics is committed to compliance with GCP, NATA, and trial protocols.

SHORT-TERM STORAGE.
Reliable Storage for Clinical Trial Sample Integrity.
nexomics offers comprehensive and secure short-term storage solutions for clinical trial samples, providing assurance and reliability throughout the research process. Our advanced facilities in Melbourne, Australia are designed to preserve sample integrity through:
- Diverse storage environments: Room temperature, -20°C, -80°C, and liquid nitrogen LN2.
- Sample tracking and active monitoring: Regular monthly sample tracking to keep your clinical trial management team, CRO partners, and clinical researchers informed of all study developments.
- Dedicated support: A direct point of contact ensures personalised service and seamless communication.

